Author: Chris Janse
- Differences between different isolates and laboratory lines of P. berghei
- Sporozoites and pre-erythrocytic development
- Erythrocytic (blood stage) development
- Fertilization and zygote development in the mosquito
- Oocyst and sporozoite development
- Additional information on different P. berghei isolates, stabilates and laboratory lines
<< previous chapter – next chapter >>
Differences between different isolates and laboratory lines of P. berghei
The characteristics of the P. berghei life cycle described below are mainly based on observations of parasites of the ANKA isolate of P. berghei and during their growth in laboratory mice or rats and transmission by Anopheles stephensi mosquitoes. The different isolates (strains) of P. berghei show many similar and stable characteristics of their life cycles. For example, all life cycle stages of the different isolates have a similar morphology and duration of development. No variation in iso-enzymes has been found and they show in general a comparable sensitivity to antimalarial drugs and other inhibitors. In addition, the genome sequence of many laboratory lines of different P. berghei isolates is highly similar (see Otto et al., 2014. A comprehensive evaluation of rodent malaria parasite genomes and gene expression. BMC Biol. 12:86).
There are however (small) differences between P. berghei parasites of different isolates and laboratory lines that influence different characteristics of infection such as the course and intensity of blood infections, gametocyte production, virulence and pathology (see Table 1 for the definition of the terms isolate, line and clone; isolates are often referred to as strains in rodent parasites).
Reported variations are mainly the result of variation in environmental factors and by differences in vertebrate or mosquito hosts used in different studies, but may also result from (genetic) differences between the isolates and laboratory lines of P. berghei. A number of well-documented variable characteristics of P. berghei are listed below. These characteristics have been largely described at the level of phenotype and, therefore, their genetic and molecular basis is unknown (see also Table 2).
- Reticulocyte preference.
P. berghei has a strong preference for invading reticulocytes. To our knowledge, no lines of a single isolate of P. berghei have been obtained in the laboratory that show stable, significant differences in preference for invading different red cell populations. There is however evidence that different isolates show ‘small’ but significant differences in reticulocyte preference. For example, some lines of the NK65 isolate have a stronger preference for reticulocytes in comparison to lines of the ANKA isolate in Swiss mice. The reticulocyte preference can also be host-dependent: we observed that parasites of the ANKA isolate have a stronger reticulocyte preference in Wistar rats than in Swiss mice. Most P. berghei ANKA lines have a strong preference for invasion into reticulocytes. During the course of an infection, when the availability of reticulocytes becomes a limiting factor, most ANKA parasite lines have the capacity to ‘switch to invading normocytes’. When reticulocytes are available again in the circulation, parasites ‘switch back’ to invading reticulocytes. In different hosts P. berghei ANKA clones show differences in the capacity to switch to invading normocytes. For example, in WISTAR rats the switch to normocyte invasion is (nearly) absent. Between experiments, or in different mice, parasites of the same ANKA clone can either switch to normocyte invasion or remain restricted to invasion of reticulocytes. Infections in mice then have two typical courses of parasitemia. Initially, all infections have a typical reticulocyte restricted course of infection until parasitemias reach 0.5-2%; mice infected with parasites that make the switch to invading normocytes than rapidly increase in parasitemia from 0.5-2% to 15-25% within 2 days (at which stage most mice will succumb to experimental cerebral malaria (ECM) in ECM-sensitive mice). In other infections (mice) parasites remain reticulocyte restricted and in these infections there is an actual small drop in parasitemia at around 3-5% parasitemia as a consequence of a shortage of reticulocytes in circulation. After this short second phase of a reduced multiplication the parasitemia then again rapidly increases, resulting from a ‘strong wave’ of reticulocyte production. In these infections mice usually do not die of experimental cerebral malaria (ECM) but eventually die from a fulminating parasitemia. This second course of infection is much less predictable as a result of different factors as mentioned above. Outbred Swiss mice that do not develop ECM usually follow a ‘reticulocyte-restricted’ course of parasitemia, whereas Swiss mice that do die from ECM make the switch to invading normocytes. Differences exist in the capacity to switch to invading normocytes between different ANKA clones. Whether these differences are ‘genetically fixed’ in these clones or are ‘reversible’ is largely unknown due to the lack of detailed analyses of (host and parasite) differences in multiple experiments and the relatively large inter-experimental variation in the capacity to switch to invading normocytes. - Number of merozoites per erythrocytic schizont.
In general, parasites growing in more mature erythrocytes produce fewer merozoites (6-12 merozoites per schizont) than those that grow in reticulocytes (12-18 merozoites per schizont).
- Sequestration of erythrocytic schizonts.
In most laboratory animals the erythrocytic schizonts of P. berghei ANKA disappear from the blood circulation and sequester in the capillaries of the inner organs, such as the spleen, lungs and adipose tissue. To our knowledge, no stable lines of P. berghei exist which show significant differences in the site or level of sequestration of their erythrocytic schizonts. However, small differences might exist between different lines in the number of mature schizonts circulating in the peripheral blood. Lines of the K173 isolate, which have been mechanically passaged for many years in laboratory rodents have more schizonts in the peripheral blood than lines of the ANKA isolate.
Ring-forms, trophozoites (young and mature) and gametocytes do not sequester.
Both the immature and mature schizonts of P. berghei ANKA (completely) disappear from the circulation in the synchronized infections and are not present in tail blood (occasionally very mature schizonts may be seen and in mice with asynchronous infections with high parasitemias schizonts are observed, although in relatively low numbers).
In most rodent hosts no significant sequestration of parasitized erythrocytes/schizonts in the brain has been observed. Schizonts preferentially sequester in the spleen, lungs and adipose tissue. Evidence has been presented that there is no relation between (CD36-mediated) sequestration of schizonts and ECM although evidence has been presented for a relationship between accumulation of infected red blood cells in the brain (at the onset of cerebral complications) and ECM. - Virulence and pathology.
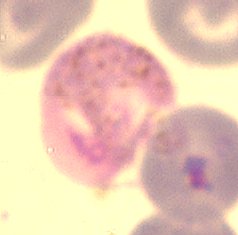 An infection with P. berghei causes death within 1 to 3 weeks in many strains of laboratory mice and rats and many animals die from cerebral complications (experimental cerebral malaria; ECM). A large number of environmental factors influence the virulence and pathology of P. berghei and as implied above the (genetic background of the) host is one of the most important factors. In most laboratory rodents P. berghei infections cause rapidly fulminating infections leading to death, whereas in the natural host (and several strains of laboratory rats, for example Brown Norway rats) P. berghei causes long-lasting, chronic infections with low parasite densities. In several mouse strains, P. berghei causes cerebral complications (ECM), but between rodent strains significant differences exist in the susceptibility to cerebral complications. For example, Brown Norway rats and older Wistar rats show no cerebral complications and are able to clear parasites from the blood and recover from a P. berghei infection. The absence of ECM in many different knock-out mutants of C57Bl6 mice that are susceptible to ECM has emphasized the importance of host-genetic factors in virulence and pathology caused byP. berghei . In addition to the genetic background, other host factors such as age and diet of the host influence the course of infection, virulence and pathology. Additional environmental factors that influence infection characteristics are the route of infection (sporozoite induced versus blood infections) and the dose of infection. In addition to the host and other environmental factors, (genetic) differences between laboratory lines of P. berghei can affect virulence and pathology. Several laboratory lines of P. berghei have been produced that demonstrate lower virulence. For example, a stable attenuated parasite line (XAT) of the NK65 isolate, derived by irradiation, shows a low self-resolving parasitemia in mice. In addition, less virulent P. berghei lines (reduced growth rate, reduced ECM) have been produced by genetic modification (by deleting genes from the parasite genome).
An infection with P. berghei causes death within 1 to 3 weeks in many strains of laboratory mice and rats and many animals die from cerebral complications (experimental cerebral malaria; ECM). A large number of environmental factors influence the virulence and pathology of P. berghei and as implied above the (genetic background of the) host is one of the most important factors. In most laboratory rodents P. berghei infections cause rapidly fulminating infections leading to death, whereas in the natural host (and several strains of laboratory rats, for example Brown Norway rats) P. berghei causes long-lasting, chronic infections with low parasite densities. In several mouse strains, P. berghei causes cerebral complications (ECM), but between rodent strains significant differences exist in the susceptibility to cerebral complications. For example, Brown Norway rats and older Wistar rats show no cerebral complications and are able to clear parasites from the blood and recover from a P. berghei infection. The absence of ECM in many different knock-out mutants of C57Bl6 mice that are susceptible to ECM has emphasized the importance of host-genetic factors in virulence and pathology caused byP. berghei . In addition to the genetic background, other host factors such as age and diet of the host influence the course of infection, virulence and pathology. Additional environmental factors that influence infection characteristics are the route of infection (sporozoite induced versus blood infections) and the dose of infection. In addition to the host and other environmental factors, (genetic) differences between laboratory lines of P. berghei can affect virulence and pathology. Several laboratory lines of P. berghei have been produced that demonstrate lower virulence. For example, a stable attenuated parasite line (XAT) of the NK65 isolate, derived by irradiation, shows a low self-resolving parasitemia in mice. In addition, less virulent P. berghei lines (reduced growth rate, reduced ECM) have been produced by genetic modification (by deleting genes from the parasite genome). - Gametocyte production.
The gametocyte production, defined as the percentage of blood-stage parasites that differentiate into gametocytes, is influenced by (unknown) environmental factors. Stable, genetic differences exist in gametocyte production between laboratory lines of P. berghei. Some P. berghei laboratory lines have lost the capacity to produce gametocytes. - Sex ratio of gametocytes.
 Evidence exists that the sex ratio (males:females) of P. berghei gametocytes can be affected by (unknown) environmental factors. It is unknown whether stable (genetic) differences in sex ratio exist between different isolates/lines.
Evidence exists that the sex ratio (males:females) of P. berghei gametocytes can be affected by (unknown) environmental factors. It is unknown whether stable (genetic) differences in sex ratio exist between different isolates/lines. - Gametocyte infectivity.
Infectivity of P. berghei gametocytes (defined as the capacity to produce oocysts in mosquitoes) appears to drop during the later stages of blood-stage infections in laboratory rodents (see also below). - The intensity of mosquito infection.
The intensity of mosquito infection, defined as the number of oocysts, is dependent on the gametocyte infectivity (see above) and the susceptibility of the different mosquito hosts used in the laboratory. Between different Anopheles species and between strains (lines) of one species large (genetic) differences have been reported in the susceptibility to support oocyst infection (see below). - Duration of development in the mosquito (sporogony).
The length of the sporogonic cycle in the mosquito is dependent on the environmental temperature. - Salivary invasion of sporozoites.
The rate of salivary gland invasion is different in the various mosquito hosts that are used in the laboratory. For example, the sporozoite invasion rate is higher in A. stephensi than in A. quadrimaculatus. - The infection rate of liver cells by sporozoites.
The infection rate of sporozoites in liver cells is different in the different species and strains of laboratory rodents. These rodents range from almost totally refractory to ‘highly’ susceptible to the development of the pre-erythrocytic stages. This level of susceptibility is not only determined by the sporozoite infection rate but can also be influenced by innate immune responses against developing sporozoites, just after invasion of the hepatocyte - Size of pre-erythrocytic schizonts.
The size and the number of merozoites in mature pre-erythrocytic schizonts is reported to be different in different host species - Chromosome size. See for a description of chromosome size variation the Genome of P. berghei
Table: Terms and definitions used for the description of malaria parasites
| Isolate (strain) | A sample of parasites taken from an infected person or animal on a unique occasion; an isolate is uncloned, and thus may contain more than one genetically distinct parasite clone (in rodent parasites isolates are often referred to as strains). |
| Line | Parasites of a single species derived from a single isolate, not necessarily cloned, which have some common phenotype, e.g. drug-resistance. |
| Clone | The progeny of a single parasite, normally obtained by micro-manipulation or serial dilution of uncloned parasites and then maintained in the laboratory. All the members of a clone have been classically defined as genetically identical, but this is not necessarily the case, since members of the clone may undergo mutations, chromosomal rearrangements, etc, which may survive in the culture conditions. |
Table: Some variable characteristics of P. berghei isolates/lines (due to genetic differences or variable environmental factors (see text for more details).
Variation influenced (caused) by: Variation in: |
Genetic differences between parasites | Genetic background of host | Other environmental factors |
| Reticulocyte preference | +(?) | + | ? |
| No. of merozoites/schizont | ? | ? | Age of host erythrocyte |
| Sequestration of schizonts: | |||
| Site: | ? | + | ? |
| Level: | + | ? | ? |
| Virulence/Pathology | + | + | Age of host; host diet; artificial infection methods |
| Gametocyte production | + | ? | + (unknown blood factors) |
| Sex ratio | ? | ? | + (unknown blood factors) |
| Gametocyte infectivity | ? | ? | Blood factors |
|
Intensity mosquito infection (oocyst number) |
? | + | Blood factors |
| Sporozoite invasion: salivary glands | ? | + | ? |
| Sporozoite invasion: liver cells | ? | + | ? |
| Size of pre-erythrocytic schizont (number of merozoites) | ? | + | ? |
Sporozoites and pre-erythrocytic (liver stage) development
The (ultrastructural) morphology of sporozoites of P. berghei resembles that of sporozoites of human parasites.
An infection starts with the bite of an infected mosquito which inoculates the haploid sporozoites in the bloodstream of the vertebrate host. Sporozoites home to the liver and invade hepatocytes. Intact sporozoites can be observed inside hepatocytes from a few minutes to several hours after inoculation. Hepatocyte invasion is mediated by the invagination of the host cell plasma membrane to form a parasitophorous vacuole that surrounds the invading sporozoite. Sporozoites can migrate through several host cells before invading a hepatocyte by the formation of a parasitophorous vacuole. Within the hepatocyte the sporozoite develops within 47-52 hour via the trophozoite stage into the mature schizont, that can contain 1500-8000 merozoites (the total number of merozoites per mature schizont can vary in different hosts). A detailed analysis of (the ultra-structural) morphology and development of liver-stages of the ANKA strain of P. berghei in Brown-Norway rats showed the following: Within 51h the sporozoite with a length of 12µm develops into a mature liver schizont with a diameter of 30µm. The merozoites formed have a length of 1.6µm, comparable with the size of erythrocytic merozoites. Mature liver-schizonts contain about 8000 merozoites. Nuclear division starts around 24 after invasion of the hepatocytes, which means that at least 13 nuclear divisions occur within a period of 26 hours. The basic pattern of merozoite formation is comparable to that observed in the erythrocytic schizonts and during sporogonic multiplication in the mosquitoes. After rupture of the liver cell the merozoites of the pre-erythrocytic schizont are released into the bloodstream where they invade red blood cells.
In P. berghei there is no evidence for a ‘hypnozoite stage’ in the liver
Hypnozoites are found in the human malaria parasite P. vivax and some other non-human primate malarias and are a ‘dormant’ (arrested) liver stage. The hypnozoite stage can persist for prolonged periods before it starts to develop into a liver schizont, resulting in blood infections after prolonged periods after infection by a mosquito.

Erythrocytic (blood-stage) development
The haploid merozoites that are released from the liver schizonts, invade red blood cells.
P. berghei has a preference for reticulocytes but can also invade mature red blood cells (small differences in reticulocyte preference can be observed between different isolates and laboratory lines of P. berghei and in different host strains and species; see above).
Asexual development
See Morphology of life cycle stages of P. berghei for light-microscope pictures of different life cycle stages.
Within an erythrocyte the merozoite develops into a trophozoite, characterized by an increase in cell size and cytoplasm. The trophozoite consumes the hemoglobin of the red blood cell, thereby producing crystals of the brown hemozoin that can be observed as the characteristic pigment granules in the cytoplasm. The development of the merozoite (via the ring form) into a mature trophozoite, just before nuclear division starts, takes around 16 hours. Towards the end of the trophozoite stage, the parasite duplicates its DNA. Replication of the DNA is followed by nuclear division, leading to a binuclear parasite. With this first nuclear division, the parasite enters the schizont stage. During schizogony, which takes 6-8 hours, the parasite replicates its DNA and divides its nuclei a number of times, forming a syncytial cell with 8-24 nuclei. Nuclear division is endomitotic, a common feature in unicellular eukaryotes, and the segregating chromosomes and the spindle apparatus remain within the nuclear envelope throughout the process. The individual chromosomes do not condense into tight, visible structures like those seen in classical mitosis. Only towards the end of schizogony does the parasite start to divide its cytoplasm by budding of small, uninuclear merozoites. Like P. falciparum, P. berghei has a plastid-like organelle that contains a circular, extra-chromosomal genome of ~30 kb (apicoplast genome). This DNA shows 70-95% homology with the 30 kb apicoplast genome of P. falciparum and the arrangement of characterized genes is similar to that found on the P. falciparum apicoplast genome. In addition, rodent parasites have a ~6 kb extra-chromosomal mitochondrial DNA, homologous to the mitochondrial genome of P. falciparum. The total duration of the asexual blood-stage development is 22-24 hours. Mature schizonts in mature erythrocytes usually contain fewer merozoites (8-12) in comparison with schizonts in reticulocytes (16-18 nuclei). In the mature schizonts, the pigment granules (hemozoin granules) become compacted in a single ‘food vacuole as a single, dense, rounded mass.
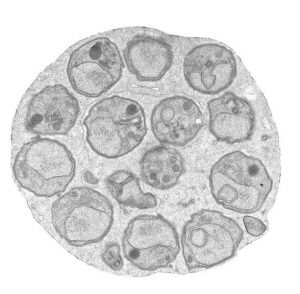 The (ultrastructural) morphology of merozoites of P. berghei resembles that of merozoites of human parasites. The merozoite has many special features related to invasion of red blood cells, such as the apical organelle (the rhoptries, micronemes, dense granules). A number of (surface) proteins of merozoites, which play a role in invasion, are conserved between rodent and human malaria parasites.
The (ultrastructural) morphology of merozoites of P. berghei resembles that of merozoites of human parasites. The merozoite has many special features related to invasion of red blood cells, such as the apical organelle (the rhoptries, micronemes, dense granules). A number of (surface) proteins of merozoites, which play a role in invasion, are conserved between rodent and human malaria parasites.
Immature and mature P. berghei ANKA schizonts disappear from the peripheral circulation and sequester in the capillaries of inner organs, such as the spleen, lungs and adipose tissue (differences in the level and site of sequestration can be observed between different isolates and laboratory lines of P. berghei and between different host strains and species). In rodent malarias the proteins on the surface of infected erythrocytes and their role in sequestration are largely unknown. No proteins have so far been described that are equivalent to the variant surface protein PfEMP1 of P. falciparum.
After rupture of the schizonts, the free merozoites invade new red blood cells, resulting in an increase in the parasitemia (= percentage of infected red blood cells).
The blood-stage development of P. berghei in laboratory rodents is usually asynchronous, which means that the different developmental stages, such as rings, trophozoites and schizonts are simultaneously present in the blood during the course of infection.
Sexual development
 See Morphology of life cycle stages of P. berghei for pictures of the different life cycle stages.
See Morphology of life cycle stages of P. berghei for pictures of the different life cycle stages.
In each asexual cycle, a small proportion of parasites stop asexual multiplication and differentiate into sexual cells, the so-called gametocytes. These haploid macrogametocytes (females) and microgametocytes (males) are the precursor cells of the female and male gametes.
In each blood-stage cycle 5-25% of P. berghei ANKA parasites are committed to sexual differentiation.
This relatively fixed percentage of cell that differentiates into sexual cells is different from the sexual development in P. falciparum where periods of ‘pure’ asexual multiplication are alternated with waves of gametocyte production.
In P. berghei, the merozoites of liver schizonts are able to differentiate directly into gametocytes after invasion of an erythrocyte.
The period of the development of a merozoite into a mature gametocyte is ‘short’ and takes only 26-30 hour.
During the first 16-18 hours of development, the ‘gametocytes’ cannot be distinguished from asexual trophozoites at the light-microscope and electron-microscope level. After 18-22 hour sex-specific features develop, such as a single enlarged nucleus, even distribution of pigment granules throughout the cytoplasm and size of the cells (filling the red blood cell). At the EM level osmiophilic bodies can be found. However, females cannot yet be distinguished from male gametocytes. Only after 24h do male-specific features become apparent, such as an enlarged, eccentric nucleus, less dense stained cytoplasm as a result of the breakdown of ribosomes and fewer osmiophilic bodies compared to female gametocytes (EM level). The developmental time of 26-30 hour is short in comparison with the developmental time of 8-11 days of gametocytes of P. falciparum. Also, the morphology of the gametocytes of these two species is different: banana-shaped gametocytes in P. falciparum and round to oval gametocytes in P. berghei. The morphology, developmental time and production of gametocytes in P. berghei show more similarity with those of gametocytes of the other human and non-human primate malarias.
It is not known when commitment to sexual differentiation takes place in P. berghei parasites.
There is some evidence that sexual commitment in P. berghei occurs in the trophozoite stage between 12 and 16 hours after invasion, directing the differentiation of these trophozoites into gametocytes. Again this would be different from P. falciparum where commitment to sexual differentiation occurs in the previous cycle prior to schizont maturation. Therefore, the merozoites of P. falciparum are already committed to becoming a gametocyte before invading a new red blood cell, while in P. berghei commitment probably occurs after invasion.
Both in P. falciparum and in P. berghei there is evidence that environmental factors influence the switch from asexual multiplication to sexual differentiation and also influence the sex ratio.
Ample evidence exists that (developing or mature) gametocytes of P. berghei do not specifically sequester in blood capillaries of the skin
Fertilization and zygote (ookinete) development in the mosquito
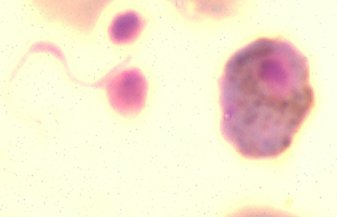
When a mosquito feeds on an infected host, only the mature gametocytes can undergo further development in the mosquito midgut. This involves an active escape of the gametocytes from the red blood cell and the formation of gametes (the process of gametogenesis). The female gametocyte differentiates into a single, spherical female gamete (macrogamete) whereas the male gametocyte produces 8 ‘sperm-like’ gametes. The formation of the male gametes is a rapid process. Within 10 minutes, three rounds of DNA replication, nuclear division and the formation of 8 flagellar axonemes occur, resulting in the production of eight, motile gametes. Three environmental triggers have been described that induce the differentiation of the gametocytes into the gametes: a drop in temperature of the infected blood to at least 5°C below that of the vertebrate host, a rise in pH from 7.3 to 7.8-8.0 and the presence of gametocyte activating factors (GAF). In P. berghei the mosquito-derived GAF is xanthurenic acid. In contrast to the distinct formation and morphology between the gametocytes of P. berghei and those of P. falciparum, the process of gamete formation and gamete morphology are highly comparable in these species. It has been demonstrated that a number of surface proteins of the gametes are conserved between rodent and human parasites. Fertilization takes place by penetration of the haploid male gamete into the haploid female gametocyte, resulting in the diploid zygote. Between 10 min. and 1 hour after the formation of gametes, fertilization and fusion of the male and female nuclei take place followed by meiosis. Meiosis is not directly followed by nuclear division, resulting in single nucleated zygote/ookinete with 2-4 times the haploid amount of DNA. The spherical
gametes. The formation of the male gametes is a rapid process. Within 10 minutes, three rounds of DNA replication, nuclear division and the formation of 8 flagellar axonemes occur, resulting in the production of eight, motile gametes. Three environmental triggers have been described that induce the differentiation of the gametocytes into the gametes: a drop in temperature of the infected blood to at least 5°C below that of the vertebrate host, a rise in pH from 7.3 to 7.8-8.0 and the presence of gametocyte activating factors (GAF). In P. berghei the mosquito-derived GAF is xanthurenic acid. In contrast to the distinct formation and morphology between the gametocytes of P. berghei and those of P. falciparum, the process of gamete formation and gamete morphology are highly comparable in these species. It has been demonstrated that a number of surface proteins of the gametes are conserved between rodent and human parasites. Fertilization takes place by penetration of the haploid male gamete into the haploid female gametocyte, resulting in the diploid zygote. Between 10 min. and 1 hour after the formation of gametes, fertilization and fusion of the male and female nuclei take place followed by meiosis. Meiosis is not directly followed by nuclear division, resulting in single nucleated zygote/ookinete with 2-4 times the haploid amount of DNA. The spherical zygote develops into a banana-shaped, motile ookinete within a period of 18-24 hour. The small pigment granules that are scattered throughout the cytoplasm of the gametocytes/zygotes become ‘packaged’ into a few clusters in the mature ookinete and are mainly located around the crystalloid (crystalloid body). Plasmodium crystalloids are transient parasite organelles that are uniquely found in ookinetes and young oocysts. Ookinetes of human and rodent parasites show a comparable morphology and like other invasive stages of Plasmodium (merozoites, sporozoites) contain an apical complex for penetration and traversing of cells of the midgut epithelium.
zygote develops into a banana-shaped, motile ookinete within a period of 18-24 hour. The small pigment granules that are scattered throughout the cytoplasm of the gametocytes/zygotes become ‘packaged’ into a few clusters in the mature ookinete and are mainly located around the crystalloid (crystalloid body). Plasmodium crystalloids are transient parasite organelles that are uniquely found in ookinetes and young oocysts. Ookinetes of human and rodent parasites show a comparable morphology and like other invasive stages of Plasmodium (merozoites, sporozoites) contain an apical complex for penetration and traversing of cells of the midgut epithelium.
Oocyst and sporozoite development
Mature, motile ookinetes traverse the midgut epithelium by invasion of cells of the epithelium and settle between the basement cell membrane and the basal lamina of the midgut wall. Most probably the P. berghei ookinete does not invade a specific midgut cell type and ookinetes traverse serially the cytoplasm of several midgut cells before entering and migrating through the basolateral intercellular space to access the basal lamina. The invaded cells commit apoptosis. Upon emerging from the epithelial cell, the ookinete makes contact with, but appears to be unable to penetrate the basal lamina. Here the parasites rapidly round-up and develop into the oocyst stage. After a growth phase of the oocyst asexual, mitotic replication results in the formation of a mature oocyst that contains thousands of daughter cells (sporozoites). The oocysts increase in size from 2-3µm in diameter to about 40µm within 10-13 days. Many features of infection characteristics in the mosquito, such as numbers of oocysts produced and numbers of sporozoites found in the salivary glands is dependent on the Anopheles species, that is used to transmit P. berghei. In Anopheles stephensi infected with the ANKA strain of P. berghei an average of 8000 sporozoites per oocyst has been
membrane and the basal lamina of the midgut wall. Most probably the P. berghei ookinete does not invade a specific midgut cell type and ookinetes traverse serially the cytoplasm of several midgut cells before entering and migrating through the basolateral intercellular space to access the basal lamina. The invaded cells commit apoptosis. Upon emerging from the epithelial cell, the ookinete makes contact with, but appears to be unable to penetrate the basal lamina. Here the parasites rapidly round-up and develop into the oocyst stage. After a growth phase of the oocyst asexual, mitotic replication results in the formation of a mature oocyst that contains thousands of daughter cells (sporozoites). The oocysts increase in size from 2-3µm in diameter to about 40µm within 10-13 days. Many features of infection characteristics in the mosquito, such as numbers of oocysts produced and numbers of sporozoites found in the salivary glands is dependent on the Anopheles species, that is used to transmit P. berghei. In Anopheles stephensi infected with the ANKA strain of P. berghei an average of 8000 sporozoites per oocyst has been recorded. Oocyst rupture and the haploid sporozoites are released into the hemocoel that will invade the salivary glands. It has been found that only 2% of the oocysts sporozoites reach the glands in the P. berghei/A. stephensi combination. The first sporozoites reach the salivary gland 13-14 days after the infectious blood meal. Sporozoites migrate through cells of the gland and exit into the extracellular secretory space where the sporozoites can persist for many weeks before being injected into a new host. An average number of 10.000 – 100.000 salivary gland sporozoites have been recovered per A. stephensi mosquito. Per bite probably only 20-50 sporozoites are delivered to the host.
recorded. Oocyst rupture and the haploid sporozoites are released into the hemocoel that will invade the salivary glands. It has been found that only 2% of the oocysts sporozoites reach the glands in the P. berghei/A. stephensi combination. The first sporozoites reach the salivary gland 13-14 days after the infectious blood meal. Sporozoites migrate through cells of the gland and exit into the extracellular secretory space where the sporozoites can persist for many weeks before being injected into a new host. An average number of 10.000 – 100.000 salivary gland sporozoites have been recovered per A. stephensi mosquito. Per bite probably only 20-50 sporozoites are delivered to the host.
Additional information on different P. berghei isolates, stabilates and laboratory lines
Below we summarize a number of (historic and more recent) observations on the (genetic analyses of) different P. berghei isolates/ stabilates/laboratory lines.
Naming of different P. berghei isolates and stabilates available from the Edinburgh collection.
To our knowledge only the Edinburgh University has a collection of (stabilates of the) original P. berghei isolates that are well described with respect to the history of the stabilates.
See chapter ‘Introduction to Plasmodium berghei‘ for the ‘Edinburgh description’ of the 6 original P. berghei isolates: ANKA, K173 (N-line, RC line), KSP11, LUKA, NK65, SP11 (line RLL) and the description of the various P. berghei stabilates of these isolates, present in the Edinburgh Collection.
Some characteristics of the Edinburgh P. berghei isolates
– The iso-enzyme composition (iso-enzyme variants of GPI, 6PGD, LDH, GDH) is the same for all P. berghei isolates (Beale, G.H., Carter, C and D. Walliker (1978) Genetics. In: Rodent Malaria (R. Killick-Kendrick and W. Peters, eds.).
– Only ‘small’ differences in karyotypes (size of chromosomes, chromosomal location of genes) have been found between parasites of the Edinburgh P. berghei stabilates.
– Analyses of gametocyte production of the Edinburgh stabilates indicate that only parasites of the Edinburgh ANKA and NK65 stabilates produce gametocytes. No gametocytes were observed in stabilates of other isolates (Leiden analyses; unpublished).
– Recent analyses of blood infections in mice of the Edinburgh lines indicate that only parasites of the Edinburgh ANKA isolate show a clear CD36-mediated schizont sequestration phenotype as described by Franke-Fayard et al. (2010; PloS Pathogens 30;6(9):e1001032). In all other lines, schizonts were observed in the peripheral blood circulation (tail blood), both at low and high parasitemia (Leiden analyses; unpublished)
Low genetic diversity between the different P. berghei isolates or ‘contamination’ of the stabilates?
Sequencing of a selected number of genes of the Edinburgh isolates has provided evidence that all these parasites, except K173 (RC) and KSP11 (RLL), may be genetically identical since they all have identical gene sequences for ama1, msp1 and dhfr. Based on these observations it has been suggested that there may be a cross-contamination that has occurred among the Edinburgh stabilates (see Saul, A. et al., 1997, Mol. Biochem. Parasitol 84, 143 – 147). Whole-genome sequencing of different P. berghei lines/stabilates from different origins/laboratories indicates that sequence diversity in P. berghei is indeed minimal (Otto et al., (2014) BMC Biology 12: 86).
P. berghei ANKA, NK65, K173 laboratory lines that are used in other laboratories
In different laboratories mainly parasites of the ANKA, NK65 and K173 isolates are used; however, the origin and history of these ‘laboratory lines’ is less well described than the Edinburgh isolates and stabilates.
ANKA: Different lines that originate from the ANKA isolate are used. ANKA reference lines from the Leiden malaria group are all generated in a cloned line using a P. berghei stabilate of ANKA, which was stored at the Institute of Tropical Medicine, Antwerp soon after its isolation from Anopheles dureni (obtained form Prof Marc Wery; Wery M., et al.,; 1979; Ann. Soc. Belge Med. Trop. 59:347–360). This cloned line, P. berghei ANKAwt (cl15cy1), has been used at the Sanger Institute to generate the reference P. berghei genome sequence. It produces gametocytes, induces experimental cerebral malaria (ECM) in ECM-sensitive mice, schizonts show a CD36-mediated sequestration phenotype and it can complete the whole lifecycle, including development in mosquitoes and in the liver. Most fluorescent and luminescent transgenic reporter P. berghei lines generated by the Leiden malaria group are made in this background parasite line.
NK65: Different laboratories use NK65 parasite lines that originate from NK65 parasites maintained and propagated in New York (see Vanderberg, J.P. et al., (1968); J. Parasitol. 54: 1009-1016). This NK65 ‘New York’ line produces gametocytes. The NK65 New York parasite lines are frequently used for studies on liver-stage infection. Other laboratories use NK65 parasites, directly derived from the Edinburgh collection (NK65 ‘Edinburgh’), which also produces gametocytes. For example, NK65 Edinburgh has been used to develop a model for malaria-associated acute respiratory distress syndrome (MA-ARDS; van den Steen et al., 2010; Am J Respir Crit Care Med. 2010, 181(9):957-68). We were unable to find detailed information on the history of the NK65 New York lines that are now used in different laboratories. Since parasites of different NK65 lines do not induce ECM in C57BL/6 mice, these parasites are often used as ‘control parasites’ in studies on ECM in P. berghei ANKA infections.
K173 parasites have been used in Nijmegen (The Netherlands) for studies on ECM in C57BL/6 mice (Curfs et al, 1990. J. Exp. Med. and other papers). This laboratory line, which has been maintained for many years by mechanical passage in mice, does not produce gametocytes and schizonts do not sequester (Franke-Fayard et al, 2010; PloS Pathogens). Interestingly, K173 parasites that have been used in different laboratories in Australia do NOT induce ECM in CBA mice (Sanni et al, 2001, Am J Pathol. and other papers). The relationship/history of the K173 Nijmegen and K173 Australia lines is unclear. The karyotype of the Nijmegen K173 parasites has been analyzed and is clearly distinct from ANKA and NK65 parasites. To our knowledge, no genotype information is available for the K173 Australia parasites.
Other P. berghei isolates
Other P. berghei isolates/lines (LUKA, KSP11, SP11) are not frequently used and it is unclear whether other institutes (other than the University of Edinburgh) have stabilates of parasites of the ‘original’ LUKA, KSP11, SP11 and K173 isolates that still produce gametocytes.
In Leiden we have analyzed parasites of K173, SP11, SP11 (RLL line) stabilates from stocks available at the Institute of Tropical Medicine Antwerp. Evidence was found for gametocyte production in SP11 and SP11 (RLL). Clones were obtained from SP11 (SP11 Antwerp clones) that produce gametocytes and schizonts show a schizont-sequestration phenotype comparable to ANKA parasites. Preliminary karyotype analyses of SP11 parasites indicate that the karyotype of these parasites is highly similar to that of P. berghei ANKA (and NK65) parasites (Leiden analyses, unpublished).
Genotyping of the different isolates/lines
Only limited information is available for the genotype (and differences in genotype) of the different P. berghei isolates and laboratory lines. To our knowledge, no diagnostic PCR or other assays have been described for genotyping the different isolates and laboratory lines. Detailed karyotype comparisons are limited. For ANKA lines/clones it has been shown that chromosomal rearrangements occur frequently during asexual propagation, resulting in significant size differences between homologous chromosomes (see the genome of P. berghei). It is therefore uncertain whether chromosome karyotyping can be used as a diagnostic characteristic to differentiate between parasites of different isolates. For example, parasites of the ANKA, NK65 and SP11 parasites show relatively similar karyotypes (Leiden, unpublished analyses). On the other hand, the ANKA Leiden and K173 Nijmegen show clearly distinct karyotypes and these differences appear to be ‘relatively’ stable.
The Sanger Institute has sequenced the genomes of parasites of the following lines i) SP11 Antwerp clone 1 (SP11 parasites obtained from Institute of Tropical Medicine, Antwerp; cloned in Leiden); ii) SP11 (RLL) uncloned (parasites obtained from Institute of Tropical Medicine, Antwerp); iii) NK65 New York (obtained from Institute Pasteur Paris) and iv) NK65 Edinburgh (obtained from the University of Leuven and cloned in Leiden, 1995cl1) v) K173cl1 (K173 parasites obtained from Nijmegen; cloned in Leiden). Only very limited sequence variation exists between the genomes of these different parasite lines (Otto et al., (2014) BMC Biology 12:86).
P. berghei isolates and laboratory lines: experimental cerebral malaria (ECM) properties
– Most ANKA lines used in different laboratories induce ECM in C57Bl/6 or CBA mice (and in most, but not all outbred MF1 and Swiss mice)
– K173 Nijmegen parasites induce ECM in C57Bl/6 (also see below)
– K173 Australia parasites do not induce ECM in CBA mice
– NK65 New York parasites do not induce ECM in C57Bl/6 (?)
– NK65 Edinburgh parasites do not induce ECM in C57Bl/6
To our knowledge, it is unknown if LUKA, KSP11 or SP11 induce ECM in C57Bl/6 or CBA mice
Is the ability to induce severe disease (ECM, MA-ARDS) a stable feature of the different P. berghei isolates or laboratory lines?
– Evidence has been presented that cloned lines of P. berghei ANKA can differ in the ability to induce ECM (Amani et al., 1998; Infect. Immunn. 66, 4093-9).
– Evidence has been presented that host diet can influence the capacity of P. berghei lines to induce ECM (Levander et al, 1995; J Parasitol 81, 99-103).
– In Leiden we have a cloned line from the Nijmegen K173 (Nijmegen K173cl1) which ‘has lost’ the capacity to induce ECM in C57BL/6 mice
– In Leiden we have observed differences in the ability to induce ECM in C57Bl/6 mice between different experiments when using P. berghei ANKA clones (see below).
– NK65 Edinburgh and NK65 New York show differences in their ability to induce MA-ARDS (Leuven analyses, unpublished).
Are other blood stage growth characteristics stable features of different P. berghei isolates or laboratory lines?
Not many features of blood-stage growth of the different P. berghei isolates/laboratory lines have been analyzed in a quantitative manner. However, in several more recent studies blood-stage growth has been compared between genetically modified mutants and their ‘wild type’ parent parasites, mainly using ANKA parasites. Blood stage growth has been determined by two methods 1) measuring parasitemia after intravenous inoculation of a single parasite during a cloning procedure or 2) measuring parasitemia after intraperitoneal or intravenous injection of 100-10.000 parasites.
In Leiden we have analyzed the growth of >1500 clones of P. berghei ANKA using method 1. Parasitemia is measured on day 7-10 after inoculation of a single parasite till a parasitemia of 0.5-2% had been reached (in Swiss mice; 25 g). More than 95% of ‘wild type’ ANKA clones show a parasitemia of 0.5-2% at day 8 which results from a (very stable) multiplication rate of 10x per 24 hour (Spaccapelo et al, Am J Pathol, 2010, 176, 205-17). Growth rates of wild-type parasites in mice with a parasitemia >2% are less predictable as there is a much larger variation in multiplication rate. This variation is influenced by a combination of factors including, but not restricted to, differences in i) ‘preference for invading reticulocytes’ (see below), ii) percentage of multiply infected red blood cells and iii) timing and production of reticulocytes by the host.
Growth and reticulocyte preference of different P. berghei isolates or laboratory lines
P. berghei parasites have a strong preference for invading reticulocytes. We are not aware of detailed studies which have compared differences in reticulocyte preference/restriction between different P. berghei isolates or laboratory lines. In Leiden we have made the following observations:
– Most P. berghei ANKA lines have a strong preference for invasion into reticulocytes. During the course of an infection, when the availability of reticulocytes becomes a limiting factor, most ANKA parasite lines have the capacity to ‘switch to invading normocytes’. When reticulocytes are available again in the circulation, parasites ‘switch back’ to invading reticulocytes
– In different hosts, P. berghei ANKA clones show differences in the capacity to switch to invading normocytes’. For example, in WISTAR rats the switch to normocyte invasion is (nearly) absent.
– In general parasites of NK65 lines have a stronger preference for reticulocyte invasion in comparison with parasites of ANKA lines
– Between experiments, or in different mice, parasites of the same ANKA clone can either switch to normocyte invasion or remain restricted to invasion of reticulocytes. Infections in mice then have two typical, different courses of parasitemia. Initially all infections have a typical reticulocyte restricted course of infection until parasitemia reach 0.5-2%; mice infected with parasites that make the switch to invading normocytes then rapidly show an increase in parasitemia from 0.5-2% to 15-25% within 2 days (at which stage most mice will succumb to ECM in ECM-sensitive mice). In other infections (mice) parasites remain reticulocyte-restricted and in these infections there is an actual small drop in parasitemia at around 3-5% parasitemia as a consequence of a shortage of reticulocytes in circulation. After this short second phase of a reduced multiplication the parasitemia then again rapidly increases as a result of a strong ‘a wave’ of reticulocyte production in the blood of infected mice. In these infections mice usually do not die of ECM, but eventually die from a fulminating parasitemia and anemia. This second course of infection is much less predictable as a result of different factors as mentioned above. Outbred Swiss mice that do not develop ECM usually follow a ‘reticulocyte-restricted’ course of parasitemia, whereas Swiss mice that do die from ECM make the switch to invading normocytes.
– We have found evidence that differences exist in the capacity to switch to invading normocytes between different ANKA clones. Whether these differences are ‘genetically fixed’ in these clones or are ‘reversible’ is largely unknown due to the lack of detailed analyses of (host and parasite) differences in multiple experiments and the relatively large inter-experimental variation in the capacity to switch to invading normocytes.
– The Leiden K173cl1 is ‘restricted’ to invasion of reticulocytes and does not switch to invading normocytes. This clone was obtained from the K173 Nijmegen line that invades both reticulocytes and normocytes. This clone does not induce ECM in C57Bl/6 mice.
Concluding remarks
As a consequence of the above-described observations of inter-clonal and inter-experimental variation, we believe that standardization between experiments is vital for analyzing genetically modified P. berghei mutants and for linking specific modifications to growth- and virulence phenotypes. In order to achieve such standardization the following aspects should be considered for analysis and reporting mutant phenotypes: (i) providing information on the origin (and genotype) of the parent P. berghei line that has been used for genetic modification; (ii) details of the host strain used (iii) determination of growth- and virulence characteristics in standardized assays, and where possible (vi) analysis of growth/virulence characteristics of two mutants that are derived from two independent transfection experiments or analysis of restoration of the wild-type genotype and phenotype in complementation experiments.
<< previous chapter – next chapter >>


